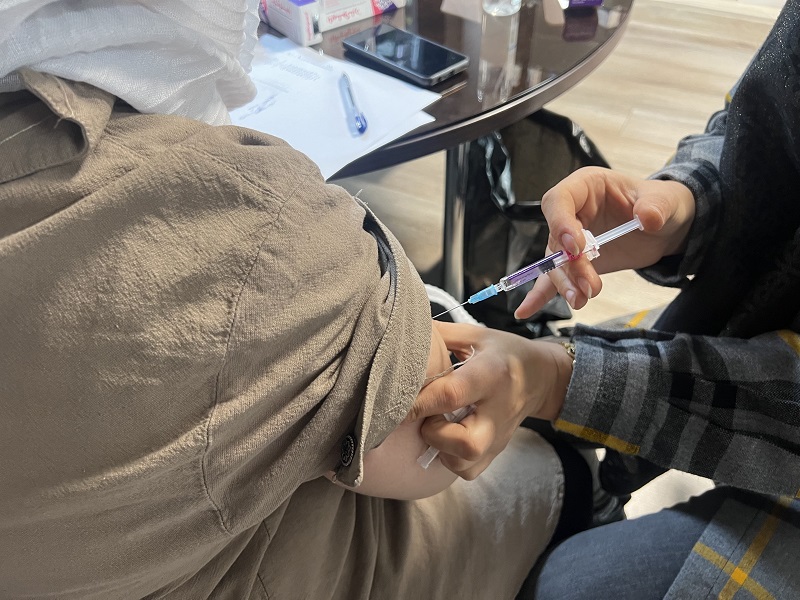
HPV vaccination for women at Hitco Holding was done to improve the safety and health of the staff. The first dose of the Papillomavirus vaccine was administered to 23 women on December 21st.
Women aged 35 to 44 are at higher risk of developing cervical cancer. More than 15% of newly diagnosed patients are women over 65 years old, especially those who have not been screened regularly. It should be noted that about 70% of cervical cancer cases are caused by HPV types 16 and 18, so vaccination plays a crucial role in preventing this disease.
The Papillomavirus vaccine is a two-dose non-combined vaccine made in the country by Novian Pezhohan Biopharmaceutical Company. The HPV vaccine is designed to prevent cancers caused by HPV types 16 and 18.
The HPV vaccination for women at Hitco Holding will continue with two more doses.
The contraindications for HPV vaccination are as follows:
Not recommended for individuals who have allergic reactions after receiving one dose of the vaccine.
The use of the HPV (Papillomavirus) vaccine is not recommended during pregnancy.
What is HPV?
The human papillomavirus or genital warts is one of the most common sexually transmitted diseases. This disease is caused by the presence of HPV in sexual intercourse. Fortunately, due to vaccination in recent years, this disease has decreased worldwide. However, many people are still not fully aware of the importance of vaccination and prevention of this disease.
HPV can lead to two types of low-risk and high-risk diseases. Low-risk diseases are non-cancerous, and high-risk types cause cancerous diseases.
Side effects of HPV vaccine:
The most common side effect in the vaccinated person:
pain and swelling at the injection site
Mild side effects reported: fever, dizziness, and nausea