HITCO Capacities
The overall policy of the corporation is propelling towards an operational framework, in which the dependencies to the third parties fades away gradually.
Meaning that, the majority of the affairs are preferred to be carried out internally, within an in house setup. In the same context, the following capacities are essential to fulfill this ambition.
STORAGE STANDARDS
We managed to elevate the standards of the warehouse, in order to be certified with the maximum score, while authorities paid a surprise visit, in April.
Health authorities investigate these warehouses who are quite strict and meticulous, around issuing the final certificate.
HITCO capacities in terms of storage facilities are well structured.
We store a multiple ranges of products, such as pharmaceutical, medical device, supplements and active pharmaceutical ingredients (API) in these warehouses.
The facilities have successfully been certified this year, similar to the precedent one.
Following the guidelines, disseminated by the ministry of health, regarding the storage of health relevant commodities, is the key and only way to go with. Meanwhile, allocating an expert team to the topic, is also impactful.
Majority of the employee contributed to achieve the certifications and approvals, during these years.
Pharmaceutical Production – Shams Abad Site
To this date, HITCO Group and the subsidiaries, have been cooperating with various well established CMOs for local/ under-licensed productions.
In order to maintain the international standards of manufacturing and a consistent GMP practice, HITCO Group is on the verge of finalizing the construction of its own facility in Shams Abad, Tehran Province.
We hope to have this site ready and operational within months. The facility is specifically designed for solid and semi-solid dosage pharmaceutical production.
Personal Care Solutions Production – Sorinet Site in Kish Island
The main objective of this merger is to internalize the overall stages of production of skin care, cosmetic and personal care products.
Sorinet is an established facility, that will become fully operational, across all capacities in Kish Island.
Contributions to the progress of the novel biotechnological solutions within the country
In addition, beside under-license manufacturing, HITCO Group sturdily believes in localizing the advanced therapeutic means as well, to facilitate serving the country & Iranian patients with complicated disorders.
In the same context, the management made the decision to invest in advanced therapies such as gene therapy, human driven immunoglobulin production and further life-saving medications.
Cara Yakhteh Tajhiz Azma for instance, is a knowledge based firm, which HITCO Group partnered up with. This firm is specialized in gene therapy, which is considered one of the leaders in the geographical region.
Supporting the localization of treatment methodologies, as well as medicinal solutions, both are among the core values of HITCO Group.
Investing in production of novel biotechnological products, is one of the priorities.
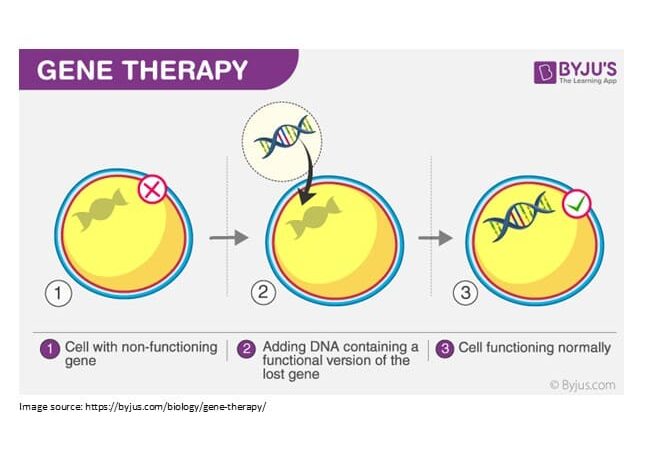
What is "gene therapy?"
Human gene therapy includes a set of novel approaches, by which the biological attributes of living cells are modified to serve a particular therapeutic need. In another word, it consists of techniques that enable advanced treatment of certain range of diseases by modifying or manipulating a patient’s gene expression.
The main area of expertise, that Cara Yakhteh Tajhiz Azma is active, would be CAR T-Cell Therapy.
How CAR T-Cell Therapy works?
This method of gene therapy, has been found to be quite effective with respect to certain type of cancers, while other treatments are no longer useful.
T-cells which contribute majorly to our immune system and it’s functionality, are capable of recognizing the foreign substances in the body. These cells detect the foreign substances through finding a certain proteins on the surface of the foreign cells, called antigens.
The immune system cells are capable of detecting and getting attached to the antigens by their receptors, Cancer cells also have antigens, but if your immune cells don’t have the right receptors, they can’t attach to the antigens and help destroy the cancer cells.
Chimeric antigen receptors (CARs)
In CAR T-cells therapies, the T-cells that are harvested from patient’s blood, that are called Chimeric Antigen Receptors (CARs). Each CAR group should be tailored for targeting a specific type of cancer, given that antigens differ in various genres of the cancer.
For instance, in certain types of leukemia or lymphoma, the cancerous cells use an antigen called CD19. The CAR T-cell therapies to treat these types of cancers are normally modified to get attached to the CD19 antigen, hence, are not applicable to a cancer that does not have the CD19 antigen.
Novin Darou: Hepatitis B & Rabies Local Immunoglobulin Producer
Novin Darou Zist Fanavar Haraz (also known as Novin Darou) was established through a blood plasma collection center in north of Iran, province of Mazandaran and city of Amol.
In fact this is a knowledge based organization, that commenced its activities in 2017. After acquiring the necessary licenses and standards, the official permission of blood collection, was issued by the MOH (Iran Ministry of Health). Today Novin Darou has managed to have an adequate plasma reserve, to produce two types of immunoglobulins, both expected to be launched during the next 4 to 6 months.
These two products include two types of immunoglobulin, for treating rabies and hepatitis B. The brands logos are indicated below:
 Hepatitis B Immunoglobulin (HBIG)
Hepatitis B Immunoglobulin (HBIG) Rabies Immunoglobulin (HRIG)
Rabies Immunoglobulin (HRIG)
To be more comprehensive, the overall procedures of treating the separated plasma and producing immunoglobulin include complex cornerstones. It is initiated from examination of the blood donor, followed by vaccination and antibody tests.
The complete scheme has been illustrated in the first figure above, from left. The efforts of young and talented scientists of Novin Darou will continue with the aim of development and commercialization of further solutions.
Darou Behboud Tamin, pioneer in development of emerging Drug Delivery Systems (DDS)
Generally speaking, there are two primary elements which we expect to improve in the science of DDS (Drug Delivery System), safety and efficacy.
The mentioned improvement is carried out via adjusting the kinetics of the drug or therapeutic agent release, plus the functionality of the system in targeting specific organ, tissue or cells with a minimized side effects.
Examples of widely used DDS types today:
- Oral Route: Therapeutic agents administrated orally, get released within the gastrointestinal tract. The release site, location or organ depend on the type of the DDS , including types of tablets, capsules, caplets or solutions.
- Transdermal Drug Delivery: This system delivers the agent into the bloodstream, through the skin. Nicotine patches are an example for this type DDS, which have helped many to quit smoking, or certain gels that are absorbed by the skin layers and enter into the blood of the person.
- Injection: Injection sterile needles are usually involved in this type of administration and consists of 3 main classifications: Intravenous (IV), Intramuscular (IM) and Subcutaneous (SC). All of the mentioned classifications offer precise control over the dosage while providing a rapid onset of action.
- Implantable Drug Delivery: This DDS was invented to replace the frequent dosing, which majority of patients find it difficult to adhere. These implants can release the therapeutic agent gradually and systematically, over an extended period.
- Nanotechnology-based Drug Delivery: Nanotechnology enables the design of drug delivery systems at the nanoscale, allowing for precise targeting and controlled release of drugs. Nanoparticles, liposomes, and micelles are examples of nanotechnology-based drug delivery systems.
There are many other emerging routes which are less common or very specific. The variety of DDS is evolving and new methodologies are being studied in medical and pharmacological universities.

Darou Behboud Tamin, specializes in nanotechnology based drug delivery systems.
Use of Micro and Nano particles in biomedicine and especially in drug delivery has a great deal of advantages over conventional systems such as: the enhanced delivery, high performance characteristics of the product, use of lesser amounts of expensive drugs in the delivery systems, extension of the bioactivity of the drug by protecting it from environmental effects in biological media, more effective treatment with minimal side effects.
As an example, the anthracycline doxorubicin (DOX), isolated from Streptomyces peucetius var. caesius, is a potent chemotherapeutic drug that is successfully used to treat various forms of liquid and solid tumors.
Unfortunately, the potential therapeutic benefits of DOX are limited by the risk of cardiotoxicity, which has been evidently related to its lifetime cumulative dose. High cumulative doses of DOX increase the probability of cardiotoxicity while individual doses are often limited by myelosuppression.
Darou Behboud Tamin has successfully formulated a liposomal doxorubicin, by applying nanotechnological means.

Recent Developments and Prospects in DDS
Click on the image for further reading about DDS